COMPUTER AIDED GUIDANCE
NE Scientific develops software for real-time simulation of ablation physics. Several ablation technologies exist, including Radiofrequency Ablation (RFA), Microwave Ablation (MWA), Cryoablation (CRA), Laser Ablation (LSA), and Irreversible Electroporation (IRE). By simulating the physics of the ablation, it is possible to determine which tissues are ablated and which not, according to the simulations, and to display this information to physicians either for planning purposes, or live during the procedure, to support and guide the procedure.
Providing an indication of which tissues are ablated and which not, as an overlay to intraoperative images during percutaneous procedures is important, as physicians do not have a direct view of the tissues they are working on, and judging which part of the tumor has been already treated and which not from images is often challenging or impossible.
The figure on the left showing a screenshot of Accublate, our ablation guidance software, in use during liver cancer ablation. The physician has a clear view of which tissues are treated and which not.
We have developed libraries for the simulation of Radiofrequency and Microwave ablation physics, development of simulation for CRA, LSA, and IRE is under way.
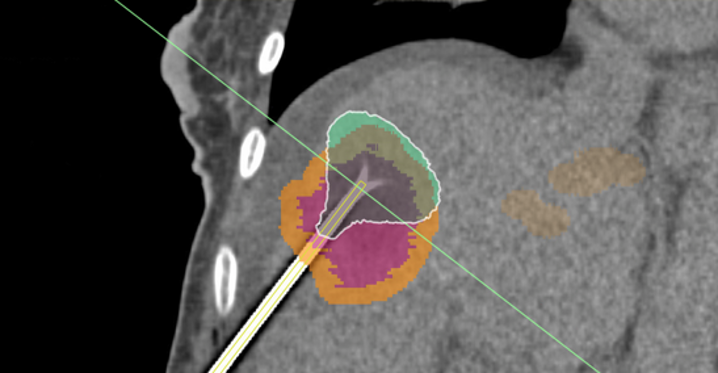
A simulation is used to provide an overlay over a CT image during the Radiofrequency ablation of a liver tumor. The ablated tissues are indicated in green, the tumor in purple and the margins (additional tissues around the tumor that need to be necrotized for safety) are indicated in yellow.
REAL-TIME SIMULATION
Real-time simulation speed is an enabling factor. Simulation of ablation physics is computationally intensive and real-time or faster simulation speeds are required in order to provide support live during the procedures.
NE Scientific has been the first company worldwide to demonstrate real-time simulation speed for Radiofrequency ablation physics in 2014, and the first company worldwide to achieve real-time simulation speed for the simulation of Microwave ablation physics in 2020, as the simulation of this modality is computationally extremely intensive.
We achieve real-time speed by carefully implementing algorithms on GPUs which are parallel computing architectures, faster than CPUs and with thousands of computing cores, as opposed to the handful of computing cores present on a CPU. The efficient implementation of algorithms on GPUs requires a deep understanding of the hardware architectures and years of experience.
In addition to using GPUs for acceleration, we have a deep expertise in numerical methods, and we have developed in house basic blocks for scientific computing, including sparse-linear solvers and ad-hoc preconditioners which, combined with GPU acceleration, allow us to achieve the simulation speeds that are needed for ablation guidance.
An NVIDIA Tesla A100 GPU features 6,912 parallel computing cores. Use of GPUs allows NE Scientific to provide real-time simulation during surgical procedures. NE Scientific is part of the NVIDIA Inception Program, a support program open to startups that make innovative use of GPU technologies.

An NVIDIA Tesla A100 GPU features 6,912 parallel computing cores. Use of GPUs allows NE Scientific to provide real-time simulation during surgical procedures. NE Scientific is part of the NVIDIA Inception Program, a support program open to startups that make innovative use of GPU technologies.
REAL-TIME SIMULATION
Real-time simulation speed is an enabling factor. Simulation of ablation physics is computationally intensive and real-time or faster simulation speeds are required in order to provide support live during the procedures.
NE Scientific has been the first company worldwide to demonstrate real-time simulation speed for Radiofrequency ablation physics in 2014, and the first company worldwide to achieve real-time simulation speed for the simulation of Microwave ablation physics in 2020, as the simulation of this modality is computationally extremely intensive.
We achieve real-time speed by carefully implementing algorithms on GPUs which are parallel computing architectures, faster than CPUs and with thousands of computing cores, as opposed to the handful of computing cores present on a CPU. The efficient implementation of algorithms on GPUs requires a deep understanding of the hardware architectures and years of experience.
In addition to using GPUs for acceleration, we have a deep expertise in numerical methods, and we have developed in house basic blocks for scientific computing, including sparse-linear solvers and ad-hoc preconditioners which, combined with GPU acceleration, allow us to achieve the simulation speeds that are needed for ablation guidance.
PATIENT SPECIFIC MODELING
The volume and shape of an ablation are determined by the energy provided, by the technical characteristics of the system in use, and by a number of patient specific factors which can alter the actual ablated volume.
As we can simulate the physics of the ablation, we can account for many of these factors in the simulations and provide an indicated ablation volume which is generally more accurate than the ablation footprint indicated by the manufacturer of the ablation system, which is generic and does not account for any patient specificity.
As an example, in thermal ablations, where temperatures in the tissues at the ablation site exceed 80 °C or more, a vessel in the proximity of the ablation, having a temperature of 37 °C, can remove heat and alter and shrink the ablation volume. Our simulation technology, if fed with the shape of the local vascular tree our simulation technology can account for the heat-sink effect of vessels and predict the abated volume based on this information.
Perfusion has a significant effect on the ablation volume. Similar to the effect of vessels, but on a microscopic scale, perfusion removes heat from the ablated tissues, and ultimately the alation volume, for a given ablation energy, will be smaller where perfusion rates are higher and larger where perfusion rates are lower. Our simulation technology, if fed with a perfusion image of the tissues, can account for it, and provide more accurate predictions of the ablated volume.
In general, as simulations are real-time, we can account for any factor that becomes available at the time of the procedure, providing more accurate guidance information.
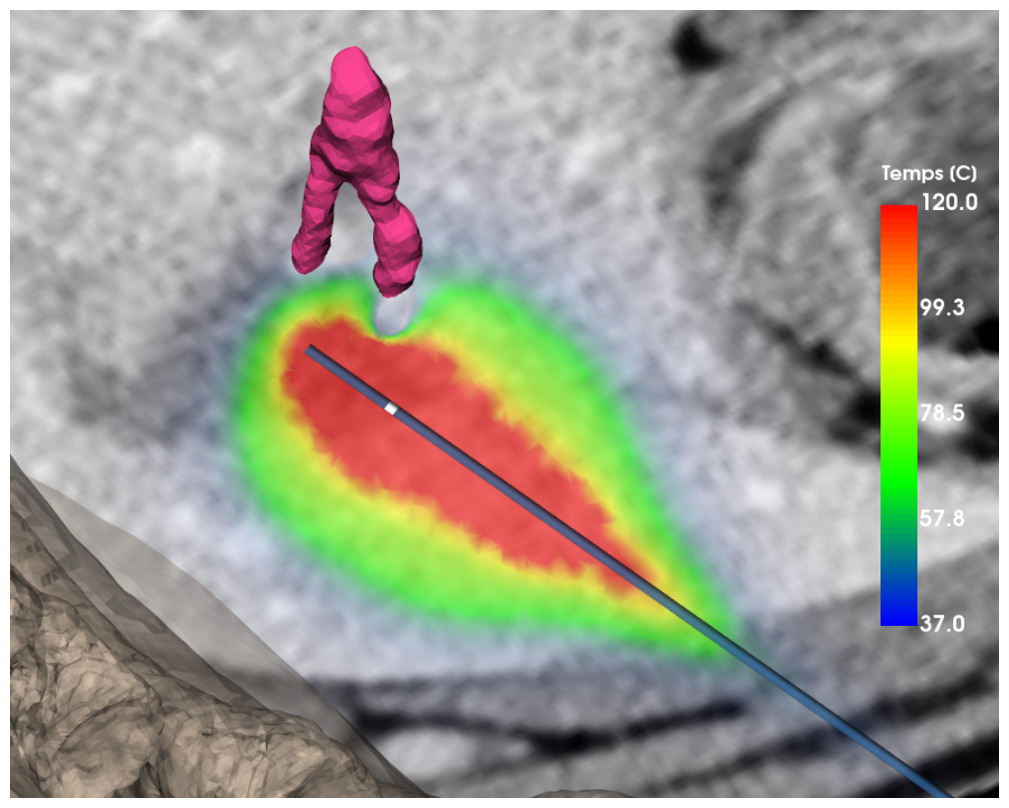
Temperatures in the tissues during a Microwave ablation. The simulation accounts for the heat-sink effect of a vessel (purple) which causes a “dent” in the ablated volume.
MULTIPLE ACTIVE PROBES
Multiple probes activated concurrently are used in Microwave, Irreversible Electroporation, and Cryoablation systems to treat larger volumes of tissues in a faster time. The ablation volume depends on the relative position of all the probes deployed in the tissues, as the probes interaction with each other, and the resulting ablation volume is not just simply the sum of the individual ablation volumes.
In using multiple active ablation probes physicians wonder most of the times what the ablation volume really is, as manufactures typically provide approximate indications of the overall volume, and those indications are valid only when the probes are inserted in a specified position relative to each other – something that is almost never possible given practical constraints of avoiding, for example, major vessels.
The Accublate technology developed by NE Scientific allows real-time prediction of the ablated volume by multiple probes, allowing physicians to see ahead of applying energy what would be the abated volume for the current positioning of the probes, to adjust the probes and see how the ablated volume would update. This functionality can greatly facilitate the use of ablations systems with multiple probes, removing the guess-work that is now involved.
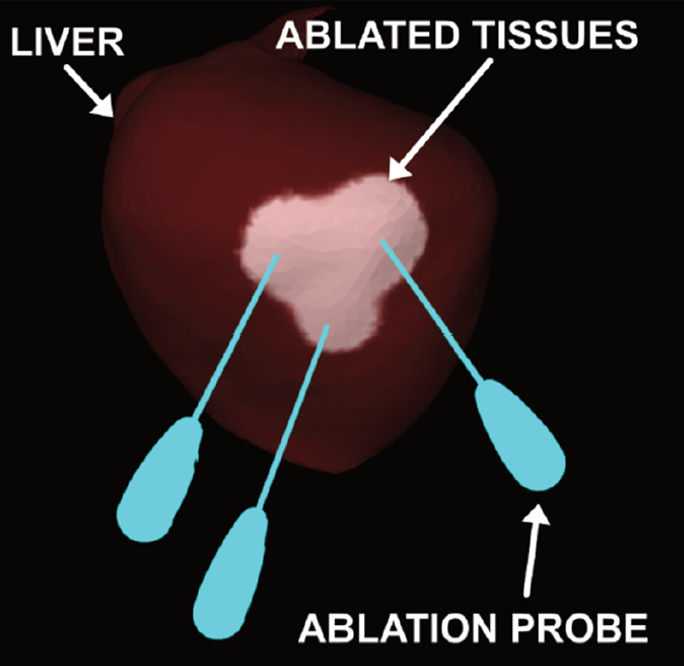
Simualation of a microwave ablation of the liver with three probes activated concurrently. The abalated tissues are shown in a lighter brown color.

Simualation of a microwave ablation of the liver with three probes activated concurrently. The abalated tissues are shown in a lighter brown color.
MULTIPLE ACTIVE PROBES
Multiple probes activated concurrently are used in Microwave, Irreversible Electroporation, and Cryoablation systems to treat larger volumes of tissues in a faster time. The ablation volume depends on the relative position of all the probes deployed in the tissues, as the probes interaction with each other, and the resulting ablation volume is not just simply the sum of the individual ablation volumes.
In using multiple active ablation probes physicians wonder most of the times what the ablation volume really is, as manufactures typically provide approximate indications of the overall volume, and those indications are valid only when the probes are inserted in a specified position relative to each other – something that is almost never possible given practical constraints of avoiding, for example, major vessels.
The Accublate technology developed by NE Scientific allows real-time prediction of the ablated volume by multiple probes, allowing physicians to see ahead of applying energy what would be the abated volume for the current positioning of the probes, to adjust the probes and see how the ablated volume would update. This functionality can greatly facilitate the use of ablations systems with multiple probes, removing the guess-work that is now involved.
AI-BASED IMAGE SEGMENTATION
NE Scientific has developed state-of-the art image segmentation libraries based on AI/DeepLearning technologies. Use of these libraries allows to extract information from pre-operative / intra-operative images and feed it to the Accublate algorithms that predict the ablated volume.
An example of information which can be extracted is the vascular tree. Contrast-CT intraoperative images can be processed to extract automatically the structure of the vasculature, which is then fed to the Accublate algorithms to estimate the ablation volume accounting for the het-sink effects.
Use of AI/DeepLearning permits an accurate extraction of body structures from 3D CT volumes with accuracy and speeds that are unparalleled with traditional image processing approaches. A typical volumetric segmentation takes 3 to 5 seconds with our DeepLearning libraries.
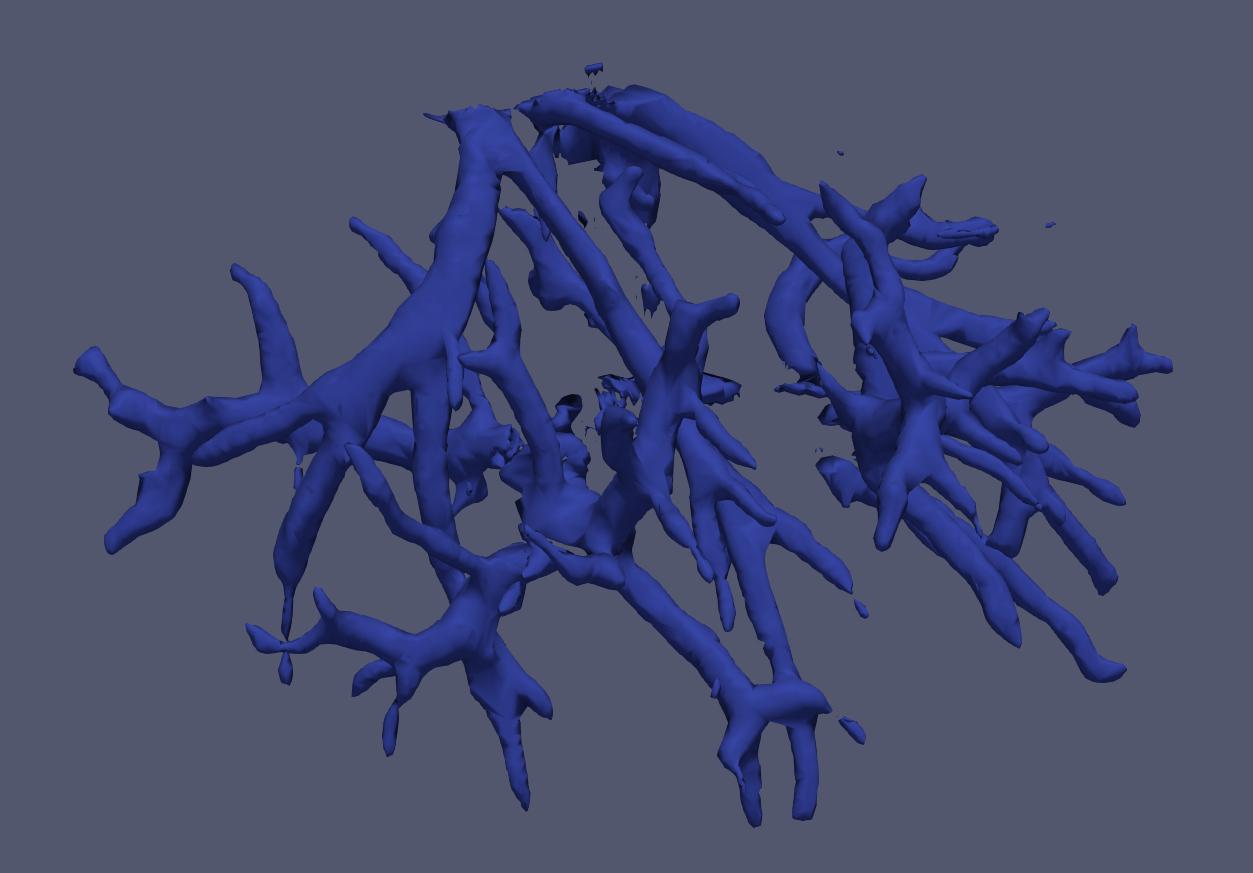
Rendering of a vascular tree in the liver segmented automatically with DeepLearning technologies developed by NE Scientific.
PATENTS
NE Scientific has filed multiple patent applications at national and international level to protect the intellectual property developed by the company.
